Visualizing cortical blood perfusion after photothrombotic stroke in vivo by needle-shaped beam optical coherence tomography angiography
doi: 10.1186/s43074-024-00124-9
Visualizing cortical blood perfusion after photothrombotic stroke in vivo by needle-shaped beam optical coherence tomography angiography
-
Abstract:
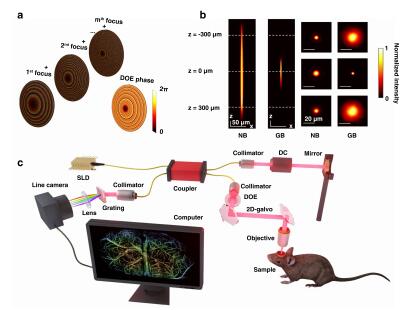
Optical imaging techniques provide low-cost, non-radiative images with high spatiotemporal resolution, making them advantageous for long-term dynamic observation of blood perfusion in stroke research and other brain studies compared to non-optical methods. However, high-resolution imaging in optical microscopy fundamentally requires a tight optical focus, and thus a limited depth of field (DOF). Consequently, large-scale, non-stitched, high-resolution images of curved surfaces, like brains, are difficult to acquire without z-axis scanning. To overcome this limitation, we developed a needle-shaped beam optical coherence tomography angiography (NB-OCTA) system, and for the first time, achieved a volumetric resolution of less than 8 μm in a non-stitched volume space of 6.4 mm × 4 mm × 620 μm in vivo. This system captures the distribution of blood vessels at 3.4-times larger depths than normal OCTA equipped with a Gaussian beam (GB-OCTA). We then employed NB-OCTA to perform long-term observation of cortical blood perfusion after stroke in vivo, and quantitatively analyzed the vessel area density (VAD) and the diameters of representative vessels in different regions over 10 days, revealing different spatiotemporal dynamics in the acute, sub-acute and chronic phase of post-ischemic revascularization. Benefiting from our NB-OCTA, we revealed that the recovery process is not only the result of spontaneous reperfusion, but also the formation of new vessels. This study provides visual and mechanistic insights into strokes and helps to deepen our understanding of the spontaneous response of brain after stroke.
-
Key words:
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Optical coherence tomography angiography /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Diffractive optical elements /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Needle-shaped beam /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Brain imaging /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Stroke /
- Engineering" /
- " data-track="click" data-track-action="view keyword" data-track-label="link">Cortical blood perfusion
-
[1] Campbell BC, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, et al. Ischaemic stroke Nature Reviews Disease Primers. 2019;5(1):70. [2] Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 2015;9:3445. [3] Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol. 2017;133(2):245–61. [4] Robinson RG, Shoemaker WJ, Schlumpf M, Valk T, Bloom FE. Effect of experimental cerebral infarction in rat brain on catecholamines and behaviour. Nature. 1975;255(5506):332–4. [5] Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture: neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616–23. [6] Kuroiwa T, Xi G, Hua Y, Nagaraja TN, Fenstermacher JD, Keep RF. Development of a rat model of photothrombotic ischemia and infarction within the caudoputamen. Stroke. 2009;40(1):248–53. [7] Lu H, Li Y, Yuan L, Li H, Lu X, and Tong S. Induction and imaging of photothrombotic stroke in conscious and freely moving rats. J. Biomed. Opt. 2014;19(9):096013- [8] Yang S, Liu K, Ding H, Gao H, Zheng X, Ding Z, et al. Longitudinal in vivo intrinsic optical imaging of cortical blood perfusion and tissue damage in focal photothrombosis stroke model. J Cereb Blood Flow Metab. 2019;39(7):1381–93. [9] Zhang H, Zhao Z, Sun S, Zhang S, Wang Y, Zhang X, et al. Molecularly self-fueled nano-penetrator for nonpharmaceutical treatment of thrombosis and ischemic stroke. Nat Commun. 2023;14(1):255. [10] He L, Huang G, Liu H, Sang C, Liu X, and Chen T. Highly bioactive zeolitic imidazolate framework-8–capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Science Advances. 2020;6(12):eaay9751. [11] Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. [12] Molina C A. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42(1_suppl_1):S16-S9 [13] Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C. Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol. 2018;18:1–26. [14] Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611–22. [15] Yanev P, Dijkhuizen RM. In vivo imaging of neurovascular remodeling after stroke. Stroke. 2012;43(12):3436–41. [16] Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95. [17] Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–4. [18] Ruan L, Wang B, ZhuGe Q, Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015;1623:166–73. [19] Wang X, Leong AT, Tan SZ, Wong EC, Liu Y, Lim L-W, et al. Functional MRI reveals brain-wide actions of thalamically-initiated oscillatory activities on associative memory consolidation. Nat Commun. 2023;14(1):2195. [20] Tayyebi S, Akhavan R, Shams M, Salehi M, Farrokh D, Yousefi F, et al. Diagnostic value of non-contrast brain computed tomography in the evaluation of acute cerebral venous thrombosis. Sci Rep. 2020;10(1):883. [21] Cao R, Zhao J, Li L, Du L, Zhang Y, Luo Y, et al. Optical-resolution photoacoustic microscopy with a needle-shaped beam. Nat Photonics. 2023;17(1):89–95. [22] Wang T, Xu C. Three-photon neuronal imaging in deep mouse brain. Optica. 2020;7(8):947–60. [23] Zhu X, Huang Q, DiSpirito A, Vu T, Rong Q, Peng X, et al. Real-time whole-brain imaging of hemodynamics and oxygenation at micro-vessel resolution with ultrafast wide-field photoacoustic microscopy. Light: Science & Applications. 2022;11(1):138. [24] Zong W, Wu R, Li M, Hu Y, Li Y, Li J, et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat Meth. 2017;14(7):713–9. [25] Burgess A, Nhan T, Moffatt C, Klibanov A, Hynynen K. Analysis of focused ultrasound-induced blood–brain barrier permeability in a mouse model of Alzheimer’s disease using two-photon microscopy. J Control Release. 2014;192:243–8. [26] Li D-Y, Xia Q, Yu T-T, Zhu J-T, Zhu D. Transmissive-detected laser speckle contrast imaging for blood flow monitoring in thick tissue: from Monte Carlo simulation to experimental demonstration. Light: Science & Applications. 2021;10(1):241. [27] Drexler W, Fujimoto JG. Optical coherence tomography: technology and applications. Springer; 2015. [28] Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. [29] Standish BA, Lee KK, Mariampillai A, Munce NR, Leung MK, Yang VX, et al. In vivo endoscopic multi-beam optical coherence tomography. Phys Med Biol. 2010;55(3):615. [30] Lorenser D, Singe CC, Curatolo A, Sampson DD. Energy-efficient low-Fresnel-number Bessel beams and their application in optical coherence tomography. Opt Lett. 2014;39(3):548–51. [31] Leitgeb R, Villiger M, Bachmann A, Steinmann L, Lasser T. Extended focus depth for Fourier domain optical coherence microscopy. Opt Lett. 2006;31(16):2450–2. [32] Tamborski S, Lyu HC, Dolezyczek H, Malinowska M, Wilczynski G, Szlag D, et al. Extended-focus optical coherence microscopy for high-resolution imaging of the murine brain. Biomed Opt Express. 2016;7(11):4400–14. [33] Ding Z, Ren H, Zhao Y, Nelson JS, Chen Z. High-resolution optical coherence tomography over a large depth range with an axicon lens. Opt Lett. 2002;27(4):243–5. [34] Zhang M, Ren Z, Yu P. Improve depth of field of optical coherence tomography using finite energy Airy beam. Opt Lett. 2019;44(12):3158–61. [35] Yin B, Hyun C, Gardecki JA, Tearney GJ. Extended depth of focus for coherence-based cellular imaging. Optica. 2017;4(8):959–65. [36] Jin L, Tang Y, Wu Y, Coole JB, Tan MT, Zhao X, et al. Deep learning extended depth-of-field microscope for fast and slide-free histology. Proc Natl Acad Sci. 2020;117(52):33051–60. [37] Wu Y, Rivenson Y, Wang H, Luo Y, Ben-David E, Bentolila LA, et al. Three-dimensional virtual refocusing of fluorescence microscopy images using deep learning. Nat Meth. 2019;16(12):1323–31. [38] Zhao J, Winetraub Y, Du L, Van Vleck A, Ichimura K, Huang C, et al. Flexible method for generating needle-shaped beams and its application in optical coherence tomography. Optica. 2022;9(8):859–67. [39] He B, Zhang Y, Meng Z, He Z, Chen Z, Yin Z et al. Whole Brain Micro-Vascular Imaging Using Robot Assisted Optical Coherence Tomography Angiography. IEEE J. Sel. Top. Quantum Electron. 2022;29(4: Biophotonics):1–9 [40] Kovács Z, Ikezaki K, Samoto K, Inamura T, Fukui M. VEGF and flt: expression time kinetics in rat brain infarct. Stroke. 1996;27(10):1865–73. [41] Kut C, Chaichana K L, Xi J, Raza S M, Ye X, McVeigh E R et al. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015;7(292):292ra100–292ra100 [42] Wei X, Camino A, Pi S, Hormel TT, Cepurna W, Huang D, et al. Real-time cross-sectional and en face OCT angiography guiding high-quality scan acquisition. Opt Lett. 2019;44(6):1431–4. [43] Li Y, Chen J, Chen Z. Advances in Doppler optical coherence tomography and angiography. Translational biophotonics. 2019;1(1–2): e201900005. [44] Nguyen VP, Qian W, Li Y, Liu B, Aaberg M, Henry J, et al. Chain-like gold nanoparticle clusters for multimodal photoacoustic microscopy and optical coherence tomography enhanced molecular imaging. Nat Commun. 2021;12(1):34. [45] Wu Z, Li L, Yang Y, Hu P, Li Y, Yang S-Y et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Science robotics. 2019;4(32):eaax0613 [46] Camino A, Ng R, Huang J, Guo Y, Ni S, Jia Y, et al. Depth-resolved optimization of a real-time sensorless adaptive optics optical coherence tomography. Opt Lett. 2020;45(9):2612–5. [47] Nguyen VP, Fan W, Zhu T, Qian W, Li Y, Liu B, et al. Long-term, noninvasive in vivo tracking of progenitor cells using multimodality photoacoustic, optical coherence tomography, and fluorescence imaging. ACS Nano. 2021;15(8):13289–306. [48] Hosseinaee Z, Abbasi N, Pellegrino N, Khalili L, Mukhangaliyeva L, Haji RP. Functional and structural ophthalmic imaging using noncontact multimodal photoacoustic remote sensing microscopy and optical coherence tomography. Sci Rep. 2021;11(1):1–11. [49] Varadarajan AV, Bavishi P, Ruamviboonsuk P, Chotcomwongse P, Venugopalan S, Narayanaswamy A, et al. Predicting optical coherence tomography-derived diabetic macular edema grades from fundus photographs using deep learning. Nat Commun. 2020;11(1):130. [50] Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. -










 下载:
下载: